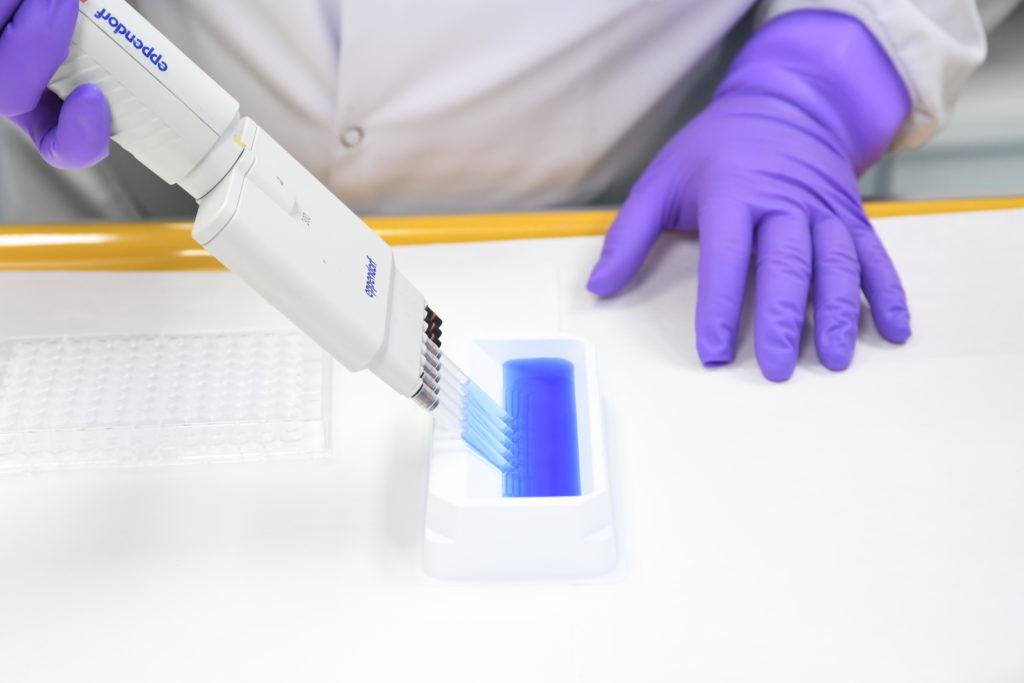
Immunoassays

Pharmaron provides in-depth immunoassay services to support global bioanalytical programs from drug discovery through to clinical trials. Our experienced team provides capabilities for pharmacokinetics (PK), pharmacodynamics (PD) and biomarker assays, immunogenicity, flow cytometry and qPCR testing.
Immunoassay Services
- Critical reagent characterization, qualification and life-cycle management for:
- Antibodies
- Peptides
- Recombinant proteins
- Biologics
- Scaffolds and matrices
- ADCs and other protein/peptide conjugates
- PEGylated molecules and fusion proteins
- Cell-based assays for drug-induced responses and detection of specific secreted molecules or proteins of interest (e.g. interleukins, cytokines)
- De novo immunoassay method development or transfer for PK/TK/ADA
- Customized biomarker assay development
- Rigorous QC/QA program and data management
- Custom designed BSL-2 laboratory including flow cytometry and cell culture suite
- Method performance and monitoring for long-term clinical projects
- Full validations per FDA/EMA/ICH guidelines for clinical PK and immunogenicity assays*
* Guidance for Industry, Bioanalytical Method Validation. US Department of Health and Human Services, Food and Drug Administration, May 2018
* Guidance for Industry, Immunogenicity Testing of Therapeutic Protein Products – Developing and Validating Assays for Anti-Drug Antibody Detection. US Department of Health and Human Services, Food and Drug Administration, January 2019
Capabilities
Our Services
Immunogenicity
- Anti-drug antibodies (ADA) assays – identification and titration of total binding antibodies
- ADA characterization for isotyping, epitope specificity and cross reactivity
- Neutralizing antibodies (NAb) assays – cell-based (primary target) and serological assessment of ADAs for neutralizing activity and NAb titers

Our Services
Flow Cytometry
- Custom assay development or assay transfer
- Specific flow cytometry (or commercial) panels:
- Biomarkers
- Immunophenotyping
- Pharmacodynamics (PD)
- Integrated clinical studies
- Phase l clinic and proximate laboratory
- Same-day sample analysis

Our Services
qPCR
- Nucleic acid extraction to target detection
- Detection of target sequences from a variety of sample types
- Validation of commercial or custom designed assays
- Viral genome detection
- Single nucleotide polymorphism detection
- Gene expression analysis

Our Services
Assay Platforms
- Molecular Devices spectrophotometer (ELISA)
- SpectraMax M2e
- Molecular Devices multimode microplate reader
- iD5
- Mesoscale Diagnostics (electrochemiluminescence)
- Meso Sector S 600
- Spectral cell analyzer (flow cytometry)
- Sony ID7000
- Becton Dickinson (flow cytometry)
- FACSCanto II
- Perkin Elmer multimode plate reader
- EnVision HTS